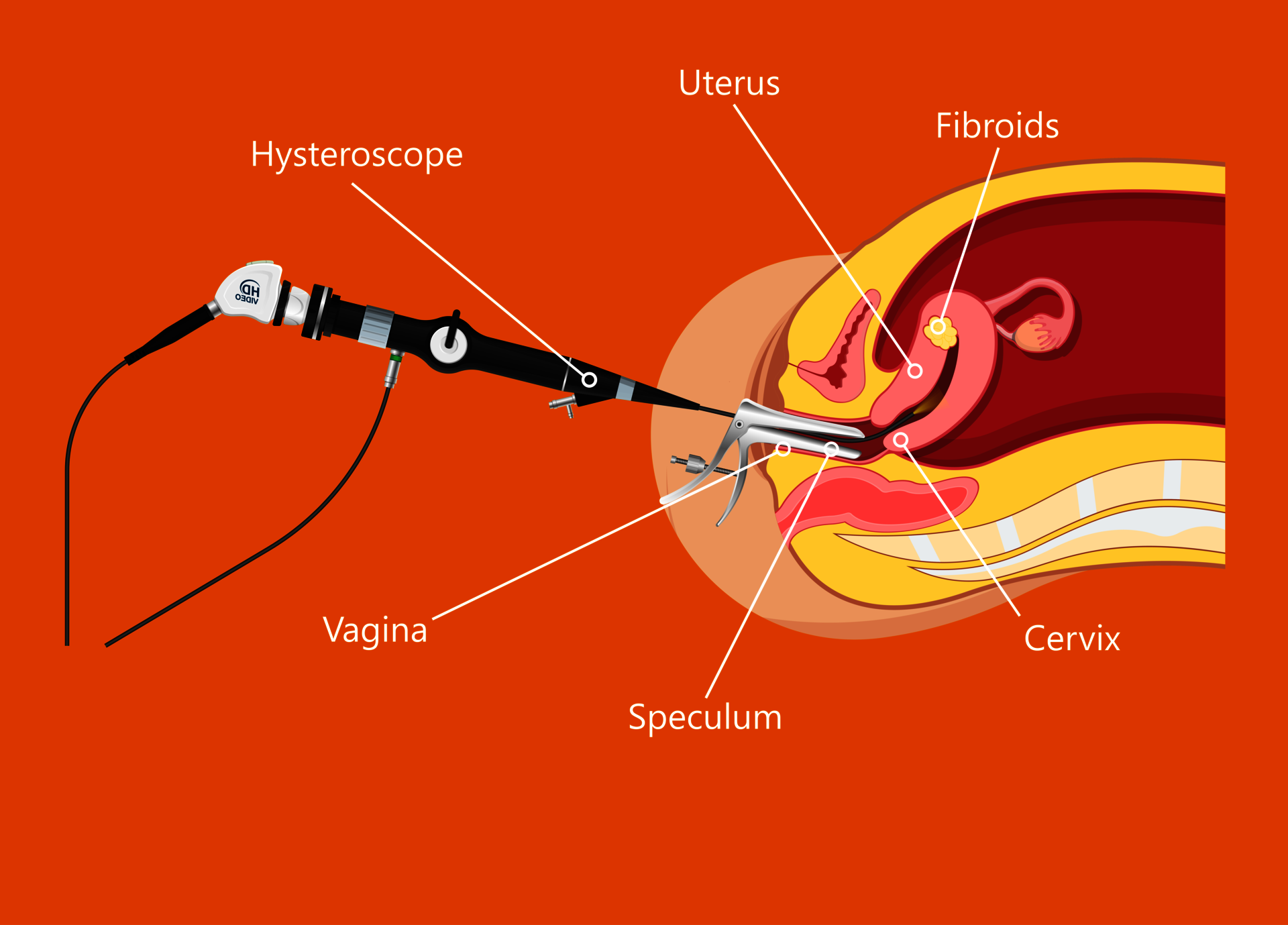
Hysteroscopyx
Why do we recommend a diagnostic hysteroscopy?
In order to clarify the cause of your symptoms (e.g. infertility or bleeding disorders), we recommend an examination of the uterine cavity, which can be combined with minor interventions. This allows changes (e.g. polyps or malformations) to be detected that cannot be clearly identified with other examination methods (e.g. ultrasound). If other examination methods come into consideration in your case, we will inform you about their advantages and disadvantages, different burdens, risks and chances of success.
Procedure
The procedure is commonly performed under (deep) sedation. The doctor inserts an optical instrument (hysteroscope) into the uterine cavity through the vagina. By introducing fluid, the uterine cavity and its walls expand. This allows changes to be precisely optically detected. Small instruments can be inserted, and tissue samples can be specifically removed and examined. Small interventions can be carried out as part of the procedure. The procedure takes up to 20 minutes averagely.
Risks and possible complications
Despite all care, complications may arise – under certain circumstances life-threatening – that require further treatment/surgery. The risk of complications (e.g. injuries/damage, bleeding, pain, fistulas, infections, allergies/intolerance) during the procedure is reported as less than 0.1% in the literature. This frequency is a general estimate and is intended to help weigh the risks. Pre-existing illnesses/signs/symptoms and individual peculiarities can of course have a significant impact on the frequency of complications.
Prior to the procedure
Please provide relevant documents. Let us know of any medication you are currently taking.
Due to the nature of the procedure, we kindly ask you to use contraceptives during the cycle in question. Sometimes as a result of elevated plasma cells in particular, certain types of antibiotics are used which can affect a developing pregnancy. If analysis of NK cells and plasma cells is also being carried out, we therefore recommend using contraceptives as well until the results of the tests are known.
After the procedure
If certain measures/precautions are required, please strictly adhere to the given instructions. Be sure to have an adult pick you up and ensure that you are looked after at home for the first 24 hours. Inform us in case of fever (over 38.5 ° C), heavy bleeding or pain.
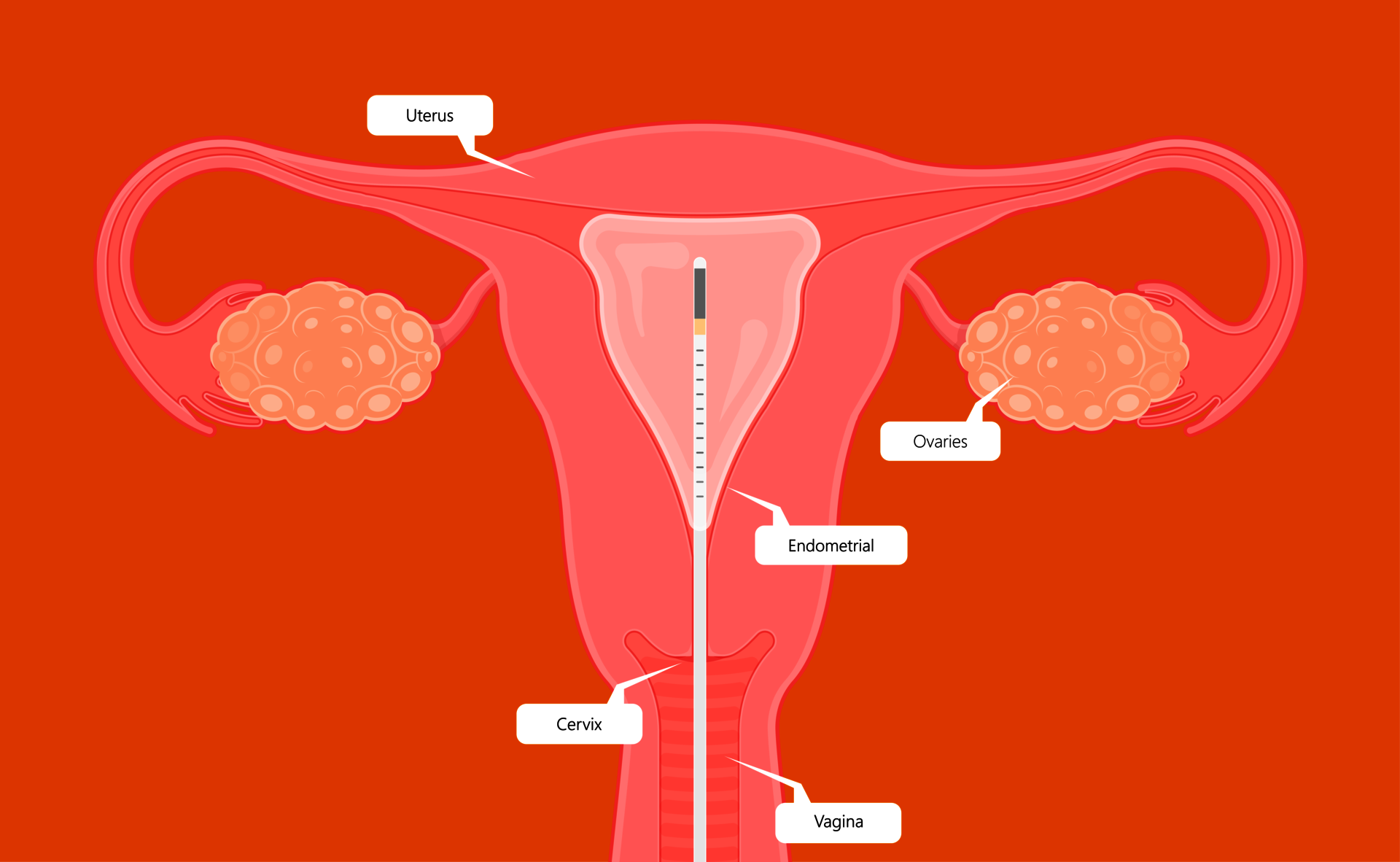
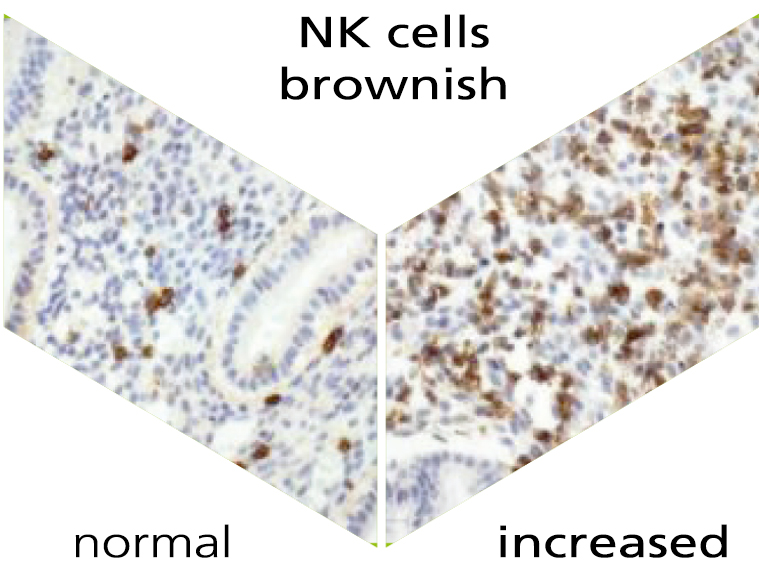
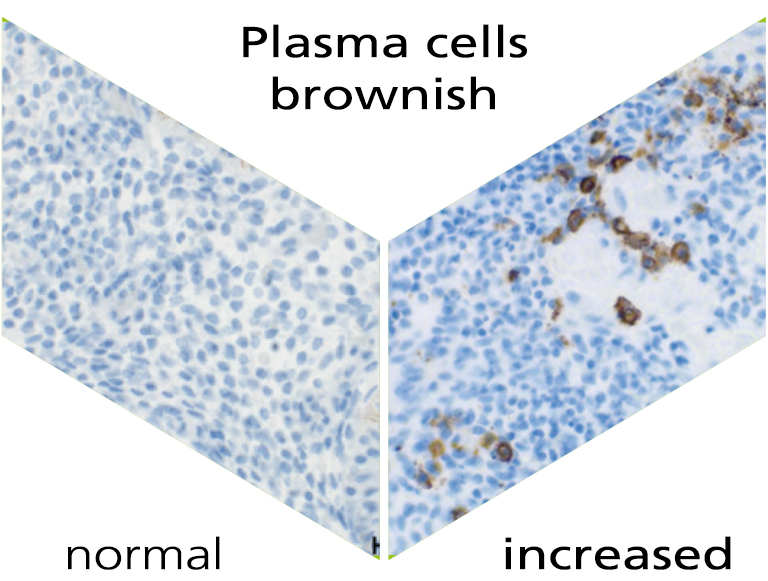
Endometrial analysis: NK cells and plasma cellsx
What are NK cells?
Our immune system plays an important role not only in everyday life, but also in pregnancy. There are various immunological factors that determine the success of a pregnancy. Natural killer cells (NK cells) and plasma cells are an important part of the innate and adaptive immune system. The natural function of the NK cells is, among other things, the recognition and defence of for instance virus-infected cells. NK cells are found both in the blood and in the lining of the uterus (endometrium), i.e. uterine NK cells. Plasma cells are activated after contact with a pathogen, produce antibodies and thus help fight an infection on site. The detection of uterine NK cells and plasma cells takes place via a specific immunohistochemical staining.
NK cells are one of the most important immune cells during early pregnancy. In the first trimester of pregnancy, 70% of the white blood cells in the placenta are uNK cells. International studies show that an above-average number of uNK cells are found in women with recurrent implantation failure and/or repetitive miscarriages. The accumulation of uNK cells in the endometrium may lead to a rejection reaction or to prevention of implantation of the embryo.
What are plasma cells?
Chronic inflammation of the endometrium (chronic endometritis) is an inflammation mostly caused by bacteria. It occurs in around 10-20% of patients with recurrent implantation failure during fertility treatment, idiopathic subfertility and in unexplained repetitive miscarriages. However, chronic endometritis is often undetected because of the mild or even non-existent symptoms. Chronic endometritis can be detected indirectly via the immunohistochemical staining of the number of uterine plasma cells.
Procedure
To examine the endometrium for uNK and plasma cells, a tissue sample ('biopsy') is required from the endometrium. This procedure can be carried out on an outpatient basis (during the gynecological examination or, if desired, during a hysteroscopy). Prior to the procedure (which commonly takes just a few minutes), a vaginal ultrasound is performed. During the procedure you may experience some mild discomfort and after the procedure you may experience mild vaginal bleeding.
It is recommended to perform the procedure between the 19th and 22nd day of your regular cycle or 5-8 days after ovulation. In exceptional cases, a diagnosis is not possible, and a new tissue sample should be obtained by means of repeating the procedure. Your gynaecologist will receive the results of the test after 2-3 weeks. The costs of the test are not covered by health insurance. Please ask us for more information. Once all of the results are available, your gynecologist will inform you if further treatment is necessary and what the options are. After a treatment for plasma cells, a new tissue sample for plasma cell determination is usually recommended, to check if the therapy was successful.

Endometrial analysis: endometrial bacterial determination in chronic endometritisx
Procedure
In the case of (persistent) chronic endometritis (e.g. despite antibiotic therapy), we offer a micro-/molecular biological determination of germs in the endometrial tissue (uterine lining). This enables us to possible detect specific pathogens causing inflammation, und thus provide a more targeted treatment. During a gynecological examination, a culture is taken from the uterine cavity using a special type of culture swab.
The costs of the test are not covered by health insurance. Please ask us for more information. Once all of the results are available, your gynecologist will inform you if further treatment is necessary and what the options are. After a treatment for a positive result, a new sample for germ determination is usually recommended, to check if the therapy was successful.

ReceptIVFity-testx
What is the ReceptIVFity Test?
The ReceptIVFity test is a new test that may help you gain a better understanding of the success, prior to your fertility treatment. This test examines whether the conditions in the uterus are suitable for the implantation of an embryo. Scientific research has shown that the composition of bacteria in the vagina (the "vaginal microbiome") is important for the chance of becoming pregnant with fertility treatment. The relationship between the different types of bacteria forms a profile that gives a high, medium or low ReceptIVFity score.
Research shows that certain conditions (less than 20% lactobacilli, more than 35% L. jensenii, more than 28% proteobacteria and/or presence of G. vaginalis) cause a low score. The test is optimized to filter out especially the low scores in order to postpone embryotransfer until a later moment when the score is in any case medium.
How does it work?
Before the start of a treatment, during a visit to the clinic the gynecologist takes a deep vaginal swab. After the collection, this is sent to the laboratory. There, your microbiome profile is determined on the basis of the collected bacterial DNA. After about 2 weeks your gynecologist will receive the results of the test. The test is easy to perform and has no side effects. However, there are costs associated with the test that are not reimbursed by the health insurer; please do not hesitate to ask us for more information.
The results
The ReceptIVFity test determines what the profile of the bacterial balance in the vagina looks like. The test can yield a high, medium and low score. With a high score, the chance of pregnancy after an embryo transfer is high (> 50%), with an average score the chance of pregnancy is approximately 25%, and with a low score the chance of pregnancy is low (approximately 5%). The results have been proven reliable for about 2 months following the test. Further research is being conducted to determine whether and how an unfavourable profile can be influenced. Ask your attending gynecologist for more information.
Obviously, a high score does not equal a guarantee of getting pregnant during fertility treatment. Other factors (e.g. egg quality, sperm quality, genetic factors in the embryo, BMI, lifestyle) are also important. The microbiome and thus your profile can change over time. Further scientific research is being conducted into drugs to positively influence the adverse vaginal microbiome in the future.

MiOXSYSx
What is MiOXSYS?
MiOXSYS is a Male infertility Oxidative SYStem, that is accurate to use, easy to use and offers the fastest oxidative stress result available today. The testsystem is intended to be used with fresh or frozen liquefied male semen samples. It is intended to be assessed in conjunction with a regular semen analysis
Why MiOXSYS?
High levels of oxidative stress have been linked to abnormal sperm function, resulting in abnormal sperm parameters and failed outcomes of fertility treatments, for instance damaging of the sperm surface, causing an abnormal morphology and impaired motility, damage to proteins which may affect enzyme function inside the cell, peroxidation of DNA and subsequent unravelling or fragmentation, and impaired fertilisation by affecting sperm capacitation and acrosome reaction.
What does the MiOXSYS measure?
Oxidative stress is a condition, where the production of reactive oxygen species overwhelms antioxidant levels. This is considered one of the major factors to be involved in damage to spermatozoa. Male infertility is a major cause of infertility and up to 25% may be due to oxidative stress. The system measures oxidative stress, through means of measuring the transfer of electrons in all known and unknown oxidants and reductants within the male semen sample. The system utilizes an electrochemical technology for the qualitative measurement (mV) of static oxidation-reduction-potential (sORP) of human semen.
For whom is MiOXSYS intended?
The testsystem can be used in cases of recurrent pregnancy loss/implantation failure, increased paternal age, cases who are classified as ‘idiopathic infertility’/‘infertility of unknown cause’, patients with a history of genito-urinary-tract infections, patients with a varicocele, and in patients with lifestyle risk factors (nicotin, alcohol).
Test procedure
The patient is asked to deposit a sample of sperm, after which a regular semen analysis will take place to determine whether a sufficient number of motile spermatozoa are present. A small sample (30 µL) is transferred to the sample application port of the MiOXSYS sensor. Once detected, the MiOXSYS analyser will begin processing the sample. A result can be expected within five minutes, after which a report will be drawn up and will commonly be shared with the patient within 24 hours. The values are normed to patient sperm concentration in order for interpretation of patients results to be reported.
Polar body diagnostics (PBD)x
What is Polar body diagnostics (PKD/PBD)?
In our centre we offer polar body diagnostics ('PKD', also called 'PBD'). This is a form of genetic testing that can be performed during an ICSI treatment. Strictly speaking, this is also possible during IVF treatment, but it is technically more difficult, and the results can be less reliable. PKD helps to detect certain hereditary/chromosomal abnormalities in eggs before fertilisation has been completed, which increases the chance of a healthy pregnancy per transfer. It is a form of selection in which fewer embryos are usually left, but which therefore gives a higher chance of pregnancy per embryo.
Who is PKD suitable for?
PKD can be used, for example, in the case of repeated implantation failure, repeated miscarriages, women with known chromosomal abnormalities ('translocations') and when age increases (and the chance of 'aneuploidy' increases). This can also be applied on request ('electively').
A key aspect of PKD is that it only provides information about the genetic contribution of the egg. Hereditary disorders that are transmitted via the sperm cannot be detected with this. In such cases, another form of embryo selection, such as pre-implantation genetic diagnosis (PGD), may be a better option. Incidentally, the largest share of the success or failure of a treatment usually lies in the hereditary factors of the embryo. If there are abnormalities in this, they come from the egg in most cases.
How does PKD work?
During a treatment, the eggs are obtained by means of a retrieval. After the ICSI and just before fertilization is completely completed (being the day after the puncture), the so-called polar bodies – small cell structures that contain a copy of the genetic material of the egg – are removed from the egg and examined in an external laboratory. The egg itself remains in our own laboratory, and of course remains usable. Over the course of a few days and usually before the transfer on the 5th day, a result can be obtained per egg showing whether there are any abnormalities and, if so, what these abnormalities are. The eggs whose polar bodies show no abnormalities, and which continue to develop into a blastocyst are then eligible for transfer and/or freezing. Please note: an abnormal result can still show a beautiful embryo (which is therefore not suitable for transfer) and a normal result can still show an embryo with a developmental arrest. In exceptional cases, not all polar bodies can be examined, or no conclusive result is obtained. It is also possible that all eggs turn out to be chromosomally abnormal and/or a result shows an abnormality that is compatible with life, but still has (serious) consequences.
What are the advantages and limitations?
PKD has the advantage that it is a less invasive and ethically less loaded technique than embryo selection via PGD. Because only the egg are examined and not the embryos themselves, it is a suitable option for people with moral or religious objections to selecting embryos. Furthermore, unlike PGD, PKD does not seem to involve 'mosaic patterns', which makes the statement about the hereditary health of the egg more reliable than PGD.
A limitation of PKD is that it does not provide information about the genetic contribution of the sperm. This however, does not seem to be the biggest problem in the end, even with limited sperm quality, when it comes to hereditarily unhealthy embryos. However, in the case of a chromosomal translocation on the male side, PKD is unfortunately not suitable, and PGD should be considered. Incidentally, unlike PKD, PGD is only permitted in a limited number of centres and is subject to medical-ethical review. This is not the case with PKD. A PKD does not replace NIPT or other forms of hereditary diagnostics during pregnancy.
Are the costs being reimbursed?
Costs of hereditary diagnostics on eggs are usually only eligible for reimbursement if the patient to whom the eggs belong, has a known chromosomal problem (such as a ‘translocation’). The exact conditions differ per insurer and per situation. In all other cases, this form of diagnostics is not eligible for reimbursement, and you must bear the costs yourself. We will calculate the costs for the collection of the polar bodies, and the external lab will send you their own invoice in due time. This may take some time.
You can gladly discuss with our doctors in a consultation whether PKD is suitable for you and what costs and reimbursement options are possible.
Please contact us for more information.